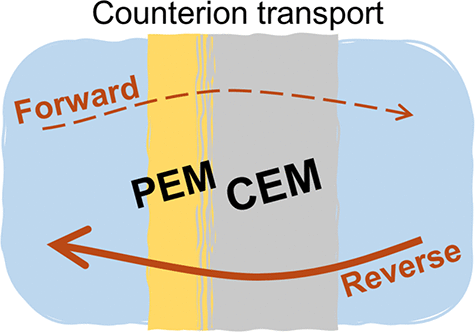
Polyelectrolyte multilayer (PEM) modified membranes can attain selective ion separations in electrodialysis with several potential applications, such as sustainable brine management. To understand the ion transport across PEM-coated membranes, we coated six different commercial cation-exchange membranes (CEMs) with PEM via the layer-by-layer technique. Coating one side of the membrane with a PEM leads to an asymmetric current–voltage response in case of solutions containing Mg2+ and Ca2+ ions. When the coating faces the counterion transport direction (FT), the coated membrane reaches a limiting current density which does not occur if the applied current is reversed. We investigated these phenomena via several electrochemical techniques. After coating, the total membrane resistance increases significantly at solutions of Mg2+ or Ca2+ (relative to the bare membrane resistance). Furthermore, the transport characteristics of the PEM coating are highly influenced by the base membrane resistance and fixed-charge density. Regarding the counterion type, the resistance of the coated membrane increases in the same order as the bare membrane: K+ < Na+< Ca2+ < Mg2+. The higher the bare membrane resistance is, the higher the PEM resistance is. The co-ion valency (i.e., monovalent Cl– or divalent SO42–) had limited to insignificant effects on the current–voltage response of the coated membranes. Therefore, dielectric exclusion is insignificant for these coated membranes at the tested concentrations, i.e., 0.25 M SO42–. Lastly, we employed an ion transport model to explain the observed effect of the current direction on the current–voltage response and analyze the effective properties of the PEM coating. Read more here: https://doi.org/10.1021/acsami.5c00155