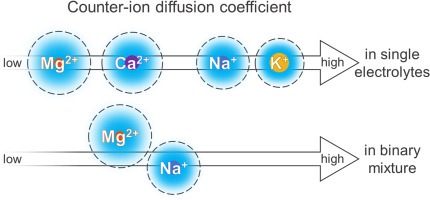
Many electrochemical technologies utilize ion-exchange membranes for water treatment (e.g. electrodialysis), energy conversion applications (e.g. redox flow batteries), and electrochemical synthesis (e.g. bipolar membrane electrodialysis). Ion mobility inside the membrane plays a primary role in determining the energy efficiency and ion selectivity of the process. We investigated the mobility of Na+, K+, Mg2+, and Ca2+ inside commercial cation-exchange membranes based on conductivity measurements in single electrolyte solutions. Moreover, we employed a transport model to simulate two scenarios for the counter-ion mobilities in a binary mixture of Na+ and Mg2+. In a single electrolyte, the mobility of various counter-ions is reduced to different extents mainly based on the membrane water volume fraction as well as the ion hydration. For example, in membranes with low-to-moderate water volume fractions, the Mg2+ mobility is 9–17 times more reduced than the mobility of Na+. In a mixture, this difference in mobility reduction is less pronounced since the ions are limited by the surrounding counter-ions inside the membranes. In this regard, the counter-ion mobilities for a single electrolyte do not necessarily reflect the counter-ion selectivity during multi-electrolyte experiments. Furthermore, the counter-ion selectivity in electrodialysis is highly influenced by the ion partitioning within the membrane in addition to ion mobilities in the diffusion boundary layer. Read more here: https://doi.org/10.1016/j.memsci.2024.123636